Why is soil pH important?
Soil pH is a parameter that growers and gardeners should take seriously. It is important for growing vegetables and fruits, but also for the flower garden.
- Many plants and organisms living in the soil have a preference for alkaline or acidic soil. By knowing the pH of the soil you are going to grow, you can select the plants that are most likely to thrive in it.
- Diseases that affect plants tend to thrive in soils with specific pH values.
- The pH value of the soil tells you the type of nutrients present in the soil.
What is pH?
Soil pH (potential hydrogen ions) is a unit of measurement of the acidity or alkalinity of the soil.
A pH value of 7.0 indicates a neutral soil (neither acidic nor alkaline).
A pH value below 7.0 indicates acidic soil.
A pH value above 7.0 indicates alkaline soil.
Soil nutrients in relation to pH
The majority of plants prefer to grow in soil that is neutral (pH=7) or slightly acidic (pH<7). As an example:
Very acidic soil (pH less than 5.4)
Eggplant, endive, potato, sweet potato, raspberry, rhubarb, rhubarb, chard, strawberry, watermelon, blueberry.
Moderately acidic (pH between 5.5 and 5.9)
Apple, basil, beans, carrots, carrots, chicory, corn, garlic, parsley, peas, peppers, pumpkin, chickpeas, turnip, turnip greens, soybeans, sunflower, tomato.
Slightly acidic (pH between 6.0 and 6.9)
Apricot, artichoke, artichoke, asparagus, beetroot, broccoli, Brussels sprouts, cabbage, dill, gooseberry, grape, kale, lettuce, mustard, mustard, okra, onion, peach, pear, spinach
Alkaline (pH between 7.1 and 8.0)
Cauliflower, celery, celery, cucumber, melon, pomegranate, thyme.
In the Plants Catalog, you can find the type of soil that each vegetable prefers.
The graph below, shows the percentage concentration of the main soil components in relation to the pH value of the soil.
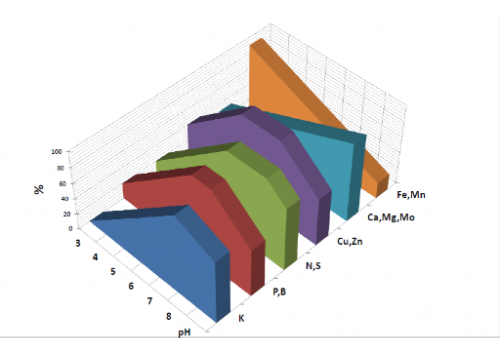
K: Potassium
P: Phosphorus
B: Boron
N: Nitrogen
S: Sulphur
Cu: Copper
Zn: Zinc
Ca: Calcium
Mg: Magnesium
Mo: Molybdenum (Molybdenum)
Fe: Iron (Iron – Ferrum)
Mn: Manganese
At this point, it should be said that there is no simple proportional relationship between the concentration of Calcium, Magnesium and Potassium in the soil and the pH values.
Which parameters influence the pH of the soil?
The pH value of the soil is influenced by the original materials that formed the soil. Soils formed from alkaline rocks usually have a higher pH. Those formed from acidic rocks have a lower pH.
The amount of rainfall also affects the pH value in the soil.
Rainwater passing through the soil leaches key nutrients such as calcium and magnesium from the soil. The leached components are replaced by acidic components such as aluminium and iron.
For this reason, soils found in places where it rains a lot are more acidic than those found in places that are dry or where it doesn’t rain much.
pH and plant diseases
Many plant diseases are caused or exacerbated by extreme pH values in the soil. This is because extreme pH values make key nutrients unavailable to plants, or because the soil itself is not healthy.
Find your soil’s pH
First of all, it’s important to say that it takes more than just one measurement to find the soil pH in your garden or field.
You need to take several measurements in different places. In the same garden, soil pH values change from point to point. These changes can be due to e.g. watering, local fertilization, plants grown, shading.
You should take measurements from several points and take an average. The average reflects the pH value of the soil.
Rough soil pH measurement – Alkaline or acidic soil?
This is the simplest and least accurate method of measurement of soil’s pH. But we can do it with materials we all have at home. You will need:
- 2 small plastic cups
- Vinegar
- Baking soda (sodium bicarbonate, bicarbonate of soda)
- Water
Fill the first glass halfway with vinegar.
Fill the second glass halfway with water and dissolve 3 teaspoons of baking soda in it.
Select the spot in the garden where you want to “measure” the pH.
Dig the soil.
Take a handful of soil and drop it into the glass with vinegar. Is it foaming vigorously? Your soil is alkaline (pH higher that 7).
Take a handful of soil and pour it into the glass with baking soda. Does it foam strongly? Your soil is acidic (pH lower than 7).
Accurate measurement of soil pH
Accurate measurement of soil pH is done by:
Electronic pH meter (pH meter)
These are small devices that look like multimeters. They have an electrode which is immersed in the soil and the pH value is measured on the pH meter. For successful measurement of soil pH, follow the manufacturer’s instructions exactly.
PH measuring strips
These are small disposable paper strips. Depending on the change in color, we find the pH value in the soil. Always follow the manufacturer’s instructions exactly.
Measurement in a laboratory
Finally, you can send samples of the garden soil to a special laboratory and get accurate measurements of the pH of the soil samples and other nutrients.
How do we correct soil pH?
The following are some techniques to raise or lower the soil pH. What you should keep in mind is that not all techniques need to be applied only once.
In order to have an effect, they must be applied regularly and there must be intermediate measurements. All techniques, try to compensate for the natural tendency of your soil to become alkaline or acidic.
How do I lower the pH of the soil?
Reducing soil pH is a slow and demanding process. In fact, trying to lower the pH of the soil goes against the tendency of the limestone in the soil to naturally dissolve and therefore raise the soil’s pH.
The most aggressive method to lower soil pH is to add rock sulfur.
To reduce the pH of sandy soil by 1 point, add 400 grams of rock sulfur per 10 square meters. For all other soils, the ratio should be 1400 grams of rock sulphur per 10 square meters.
Alternatively, you can add compost or pine needles to the soil.
Adding compost, reduces the pH value of the soil more slowly, but it has the advantage of making the soil softer and richer in organic matter, thus more fertile.
How do I raise the soil pH?
Adding limestone is the simplest method for raising soil pH. Limestone consists of either calcium carbonate (chalk) or magnesium carbonate.
Add 2.5 kilograms of limestone per 10 square meters in the fall to increase the pH by 1 point. The limestone is slowly absorbed by the soil.
By adding in the autumn we take advantage of the rains and give the soil a chance to absorb the limestone until early spring when the growing season starts.
With the addition of wood ash. Wood ash is very fast acting. You need a little because it raises the pH a lot. Use 1 kilogram for every 10 square meters.
With the addition of lime or dolomite. The amount of lime or dolomite needed to reduce the acidic pH varies depending on the soil.
Soils with high organic matter and clay are more resistant to pH changes and require larger amounts. Limestone is added to the soil to raise the pH level because it is essentially calcium, and calcium reacts with water in the soil to create hydroxyl ions.
To raise the pH of the soil by 1.0 unit:
Sandy soil: add about 140 grams of hydrated lime per square meter.
Clayey soil: add 280 grams of hydrated lime per square metre.
Clayey loamy soil: add 420 grams of lime hydrate per square metre.
Peaty soil: add 700 grams of lime hydrate per square metre.
Sources
https://en.wikipedia.org/wiki/Soil_pH
https://www.thegardenhelper.com/acidsoil.html




